As the terminology implies, Biogeochemistry is a borderline specialisation involving three basic branches of natural, physical and earth sciences. On one hand living organisms are involved and on the other substances such as nutrients, are made available to the living organisms either through soil (the pedosphere) or through water (the hydrosphere). Of course there are certain organisms which can get their food through air (atmosphere) also. The living organisms, while differing vastly among themselves in size, morphology and physiology, share, all the same a basic feature common to them all : ability to metabolise their requirements in the habitats they live.
An individual organism is perishable but life process, sustained by successive generation, is perpetual. Biogeochemistry is all about impact of such living processes on our environment. The bulk of living matter is concentrated in the landmass of the world though at the continent-ocean interface and at the bottom of the sea they are present in abundance. The physio-chemical interaction between organisms and the land that sustain them have been a subject of study for over a century now. More than two hundred years ago, Lavoisier (1743- 1794) published a treatise The turnover of elements on the surface of the globe in which he postulated on the cyclic exchange of elements among the three kingdoms of nature, namely mineral, vegetable and animal (Dobrovolsky, 1995). Lavoisier paid a heavy price for stating so : he was executed after his work was published. The interaction among the above three kingdoms essentially constitute the broad field of Biogeochemistry as we know today.
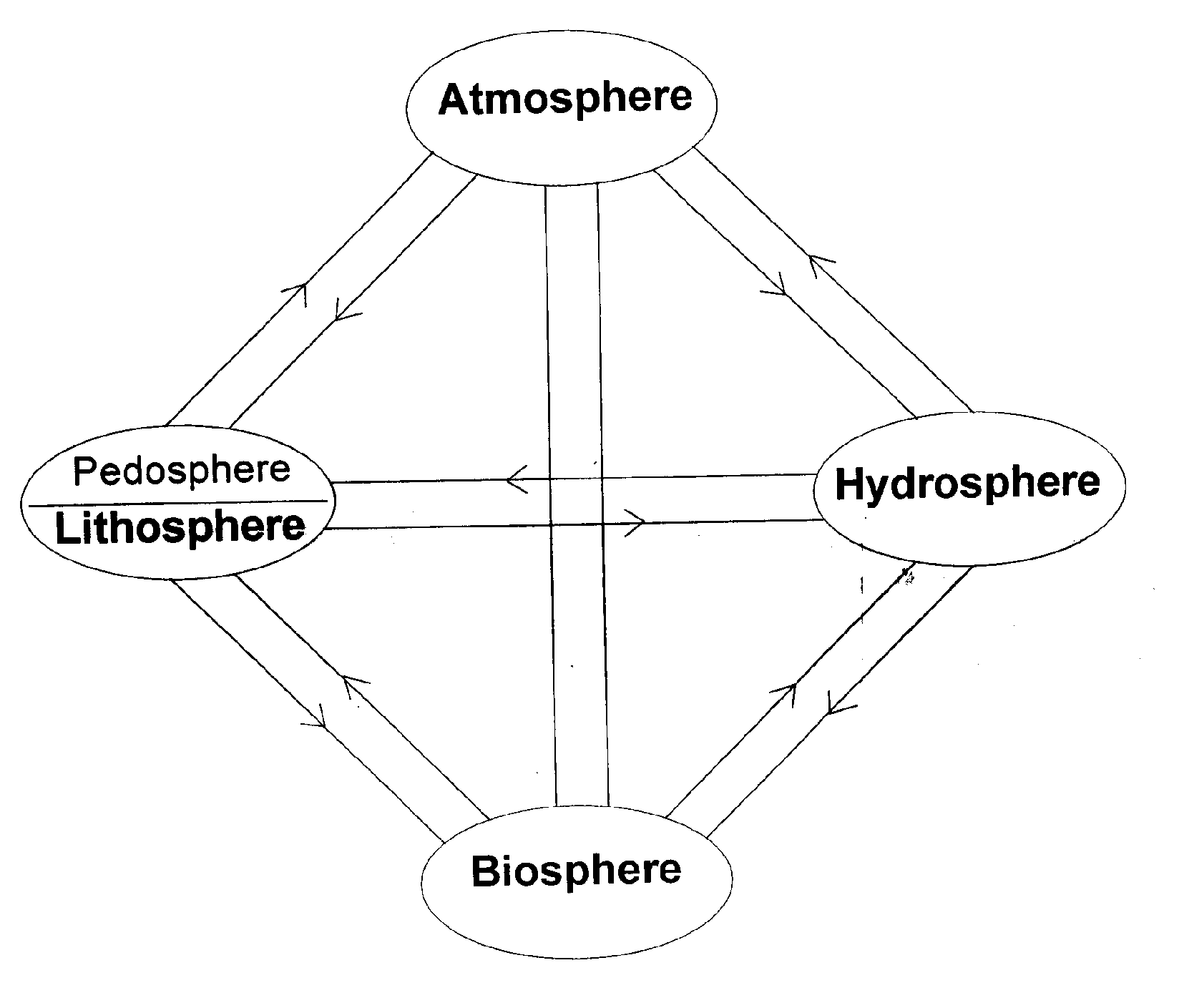
Figure-1 illustrates such interaction schematically. In this figure the original kingdoms have been replaced by reservoirs of different kind and atmosphere is brought into the picture because of gases involved in many biogeochemical processes. Since life is cyclic, all processes where living matter is involved has also got to be cyclic. Hence, in figure-1 all the reservoirs are linked to each other in both directions :at the inlet and outlet point for each reservoir. The interconnection of all the reservoirs leads to a overall global cycle. Such a cycle is known by various names depending on the user and the sector of interest : biogeochemical cycle, hydrological cycle, geochemical cycle and rock cycle. The emphasis in each cycle varies : in hydrological cycle, one will be interested in transfer of water; in the geochemical cycle, one will be interested in transfer from the crust to other segments and in rock cycle, the interest will be transformation of large volume of crustal materials across various interfaces. Whereas in the biogeochemical cycle the focus is on nutrients essential for the life processes and at the toxic substances determinant to such living things. Thus biogeochemical processes can be defined in three stages:
Stage-1: Elements to be grouped according to the needs :Essential, such as Carbon (C), Nitrogen (N) and Phosphorous (P)
Minor, such as Sodium (Na), Potassium(K) etc.
Trace, such as Iron (Fe), Manganese (Mn), Copper (Cu) etc.
Toxic, such as Mercury (Hg), Selenium (Se), Cadmium (Cd) (all the above groupings according to the requirements of biological system in the biological world) - the bio part of the topic.
Stage-2: The availability of the above from the earth, generally from the top few metres of the earth (the geocomponent, more specifically the pedosphere the geo part of the topic and now.
stage-3: The physio-chemical mechanisms that make the needs of the biological systems from the pedospheric layers of the earth in response to micro- environmental functions such as pH (acidity), weathering and release of elements by their breakdown due to chemical interaction of individual minerals with water. This constitutes the chemical part of the topic.
Let us illustrate this linkages using an essential element Carbon as an example. Referring to Figure-1, carbon is present in the atmosphere mainly as carbon dioxide (CO2) and in small quantities as carbon monoxides (CO). In the hydrosphere, carbon is present as bicarbonate (HCO3-1) and carbonate (CO3-2) the major factor in the hardness of water. In the lithosphere- pedosphere, it is present as calcium carbonate (CaCO3) or some combination thereof.
In the biosphere, carbon is present as the building block of the plants and animals in the form of variety of carbon compounds such as carbohydrates, proteins and amino-acids. Carbon enters the biological system either through geological process (weathering of carbon-bearing minerals such as limestone (CaCO3) and /or chemical process directly from the atmosphere (CO2) by photosynthesis. When the organism (including plant) dies out, the carbon is released back either directly to the atmosphere as CO2 or is mineralised as coal, limestone etc. So that it is returned to the lithosphere. Thus the carbon cycle is complete. This is illustrated in figure-2.
The oldest carbon-bearing organic world remains known to man is about 3.5 billion years whereas the age of the earth is at present estimated to be about 4.5 billion years. In spite of the complexity of the biological evolution through geological time scale, carbon has been and is being cycled through various reservoirs shown in figure-1 relatively unaffected and un- disturbed till recent times.
Among the three essential nutrients for life phosphorous (P) is extremely interesting. Unlike carbon and nitrogen, it is predominately derived from the crustal top of the solid earth and mobilised through the biosphere by the transport behaviour in the hydrosphere. Discovered in 1677, P ignites in air but glows when left in darkness. Thus it is a carrier of energy in all living systems. The homologous elements P and N has complimentary properties in relation to Hydrogen(H) and Oxygen(O2)- the other important elements for most living matter on the surface of the earth. P in association with O2 introduces some structural order in the molecules at cellular levels and thus the P-O association in the form of PO4 units separates life from ordinary inanimate minerals represented by similar association of Silicon with O2 (SiO4) (Degens, 1989).
From a single cell amoeba some 3.5 billion years ago (3500 million), organisms progressively went through complex biological evolution as a function of geological time scale. The link between time and evolution is established through changing environmental parameters such the carbon dioxide (CO2) levels in atmosphere prevailing at a given time, the O2 levels, nutrients such as P and minor and trace elements etc. Geochemical evidences through rock records show that about 2.5 billion years ago, the atmospheric CO2 was at least 100 times lower as that of today and O2 levels negligible compared to today s environment. Hence the various segments shown in figure-2 have been continuously interacting among each other through such a vast time scale to maintain the cyclic behaviour of nutrients and other elements. Excessive constituents in any one segment was removed over a period of time to other segments so that its biogeochemical cyclic behaviour was not affected. For example, during Gondwana times (about 400 to 500 million years before) when India, Australia and Antarctica were neighbours, excess CO2 in the atmosphere was neutralised by enhanced photosynthesis and subsequently the biota decayed and gave raise to most of the coal deposits which we are exploiting today. Thus due to the biogeochemical behaviour, nature was able to adjust itself at its own rate in any parameters. On the other hand in modern times, man introduces any one variable to a segment in figure-1 very abruptly and in such large dose that nature is unable to adjust to that change. A case in point the emission of green house gas CO2 by the burning of coal. The essential nutrient element C is transferred from the lithosphere to the atmosphere so fast that the excess CO2 is unable to be removed equally faster by the hydrosphere leading to enhanced levels of CO2 in the atmosphere and hence we hear everyday about the global warming (Fifure-3). On the other hand, let us take a element such as Mercury (Hg) considered to be very toxic to the biological world. Figure-4a illustrates the movement of Hg in cyclic fashion through our environment. Hg generally occurs in minerals in association with sulphur in the form of mercury sulphide (HgS) systems.
Even in rocks, it is considered to be trace element since its abundance in the crustal earth is very low. However, during the exploitation of base metals resources, such as that of copper, lead and Zinc, mercury is extracted as a buy-product. Modern civilisation uses mercury for a variety of products such as chemicals, coatings on seeds etc. Even though it does not react with water in the hydrosphere easily, mercury combines with any organic substances such decayed plant debris etc and immediately becomes toxic to the living biota. The well known minemata decease in Japan was the result of such affinity of mercury for many biotic materials. Being the only metal known to man to be in liquid state at room conditions, it easily vaporises and hence get also into the atmospheric segment of figure-4b. Hence due to man s use, what was once locked up safely in inert geological materials in rocks, have been mobilised to the biosphere, hydrosphere and atmosphere. The story is basically same for a few other toxic metals such as Cadmium though the details may individually vary due to their differing biogeochemical behaviour in our present environment.
It is clear from the above discussions that processes in our environment are better understood by focussing on the interfacing areas. Thus Biogeochemistry is the ideal such frontier area of study and research. It is however a relatively new field and only in the last two decades various researchers across the world have been focussing their attention on this inter- disciplinary subject. Outside India there are a few places where separate institutes exists for this purpose. Mention may be made of, among them to the Institute of Biogeochemistry at the University of Hamburg in Germany started by the well known late Professor Egon Degens. In India the university Grants Commission recognised the importance for strengthening research in this field and set up a special assistance programme in Biogeochemistry in the School of Environmental Sciences, Jawaharlal Nehru University in 1994 under the supervision of Prof. V. Subramanian initially for a five year period. At about the same time the Ministry of Environment and Forests also approved the setting up of a ENVIS centre in JNU on the same subject under his direction. Though there no other specific institutions in India in this subject, there are individual researchers in many universities working on topics of interest to Biogeochemistry. Mention may be made of Banaras Hindu University; Indian Agricultural Research Institute ; Garwal University; G.B. Pant Institute of Himalayan Ecology, Almora; Anna University, Madras; Annamalai University, Tamil Nadu; Dayalbagh Educational Institute, Agra; Bose Institute, Calcutta etc.
Thus, the area of Biogeochemistry is an emerging branch of multi-diciplinary approach involving all major branches of Sciences. From a humble beginning two decades ago, now this subject is well established in the international field and in India is fast catching up as an important tool of research for understanding processes in our environment. Sufficient infra- structure has now developed in India through a variety of individual and institutional efforts so that in the years to come the importance work on Biogeochemistry will be better understood and appreciated.
The involvement of a number of specialists is obvious when ones looks into the above three stages: for understanding the biotic behaviour, one should have a good understanding of ecological principles; the requirements for the ecosystem is provided by basic geochemical processes of weathering helped by microbiological and biochemical reactions in soils and over rocks; farther, from the source to the receptor, chemical and hydrological principles operate as a middleman. Hence within the broad field of Biogeochemistry itself, there are sub-specialisations depending on the background of the researchers- be a ecologist, geochemist or chemical hydrologist. Hence the word Biogeochemistry is truly an integrating field to better understand our present, past and future environmental problems.
Among the more recent sub-specialisations within Biogeochemistry that have emerged in the international research is the application of certain biochemical and molecular biochemical techniques to understand our present and past environment. A case in point is the study about the Alpine-man. A full human body was found in tact in the frozen alps and several investigations on the body using most modern biochemical techniques have thrown some light about the habitat of the community some 5000 years ago, then the prevailing eco- system, palaeo-environment, anthropological lineage etc. DNA based study thus offers excellent scope even in environment related matter. In India, a student from our laboratory is working under the supervision of Prof. Laljit Singh, Centre for DNA Finger-printing at Hyderabad to apply DNA techniques in fossils bones of geological and anthropological importance to understand the habitat and palaeo-environment. Most recently, Ministry of Environment and Forests have funded a project to two teachers of Jawaharlal Nehru University to extend DNA techniques to ancient biological specimens. Farther, characterisations of amino-acids in ancient sediments have been successfully used to understand palaeo-environment and also stresses acting on such systems. In India, however, this has not been attempted so far. Thus, Biogeochemistry can be viewed as a very challenging area of study involving a variety of basic sciences so that it is ideally suited to understand our environment better. Research should not go in the direction of reinventing the wheel but improve and if possible, replace it. Hopefully the 21st century will see consolidation of the field of Biogeochemistry as an established brach of science.

Figure 1 : The various spheres shown are broad receptors and donors to other neighbouring spheres. The Connectivity arrows, though not to scale here, Have both vectoral and scalar properties. Not all arrows have equal importance for symplifying the generalisation some sub-spheres and arrows are not shown.
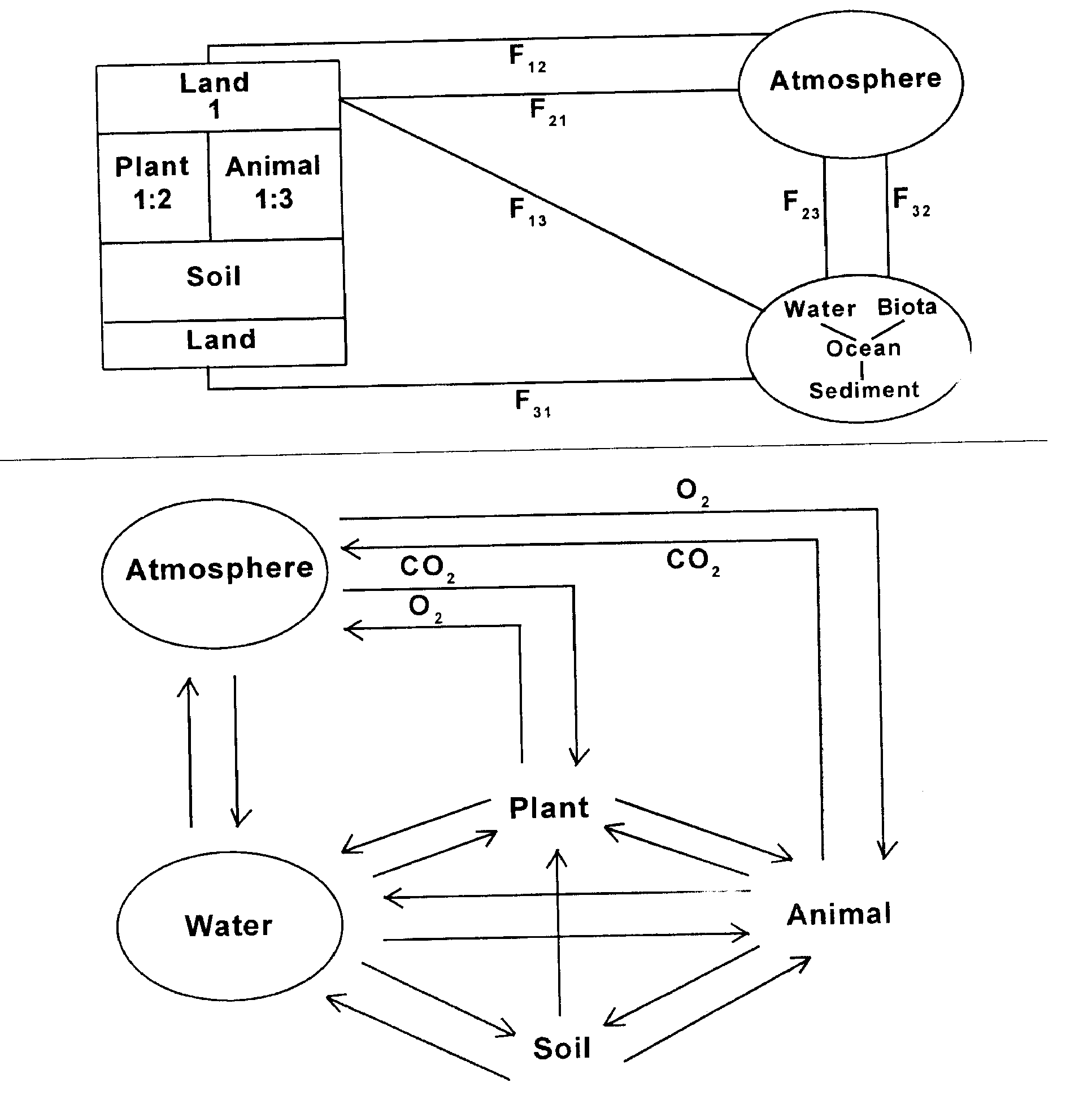
Enlarged version of Figure-1 with specific component (such as O2, CO2) various spheres/sectors, whenevr possible. Top figure shows important components in each segment and direction of transfer (e.g. F13 means transfer from 1 to 3) F means flux that is concentration for a given parameter; for ex. the volume of water/air transferred in that direction per unit time. This, F has unit of wt/vol/time. In the lower Figure, F is likely to have volumes since the time involved is relatively shorter for some of the sectors. For eg. some of the variables transferred involving soil and plants/animals and plants and atmosphere etc. may have a time scale of a few seconds only.
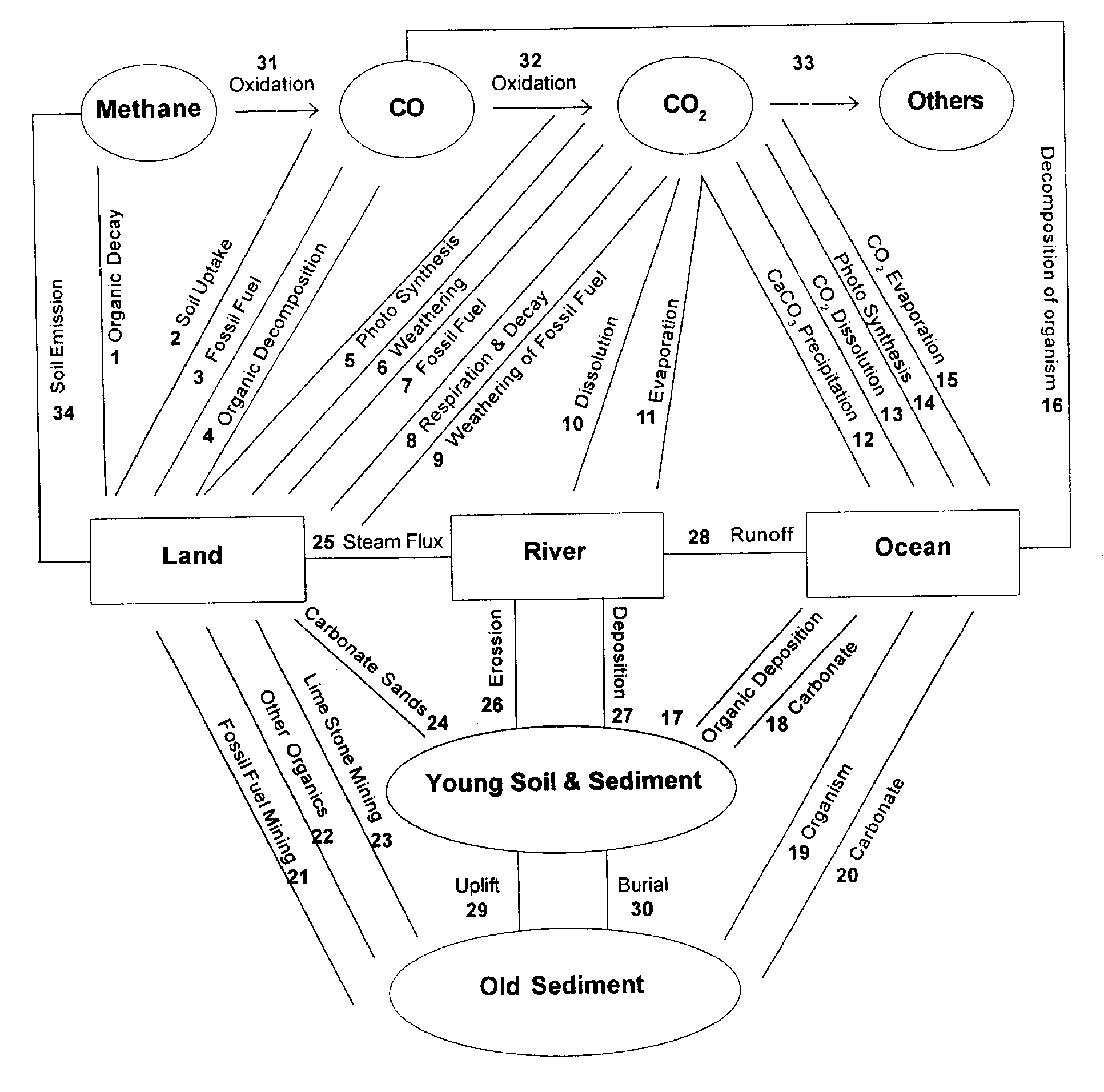
The carbon transfer, the following fluxes have been incorporated: 1 to 34 point all have same magnitude, time scale and importance. For example between river and young soils/sediments, the transfer 26 and 27 represent deposition and erosion processes. We know well by now that in modern time erosion id faster compared to deposition or accumulation so that net transfer is loss of carbon (F26>F27) from soil (Pedosphere) to river (hydrosphere). Similarly, due to deforestation, evaporation (F11) may be higher than the net river volume(F11>(F25-F28)).
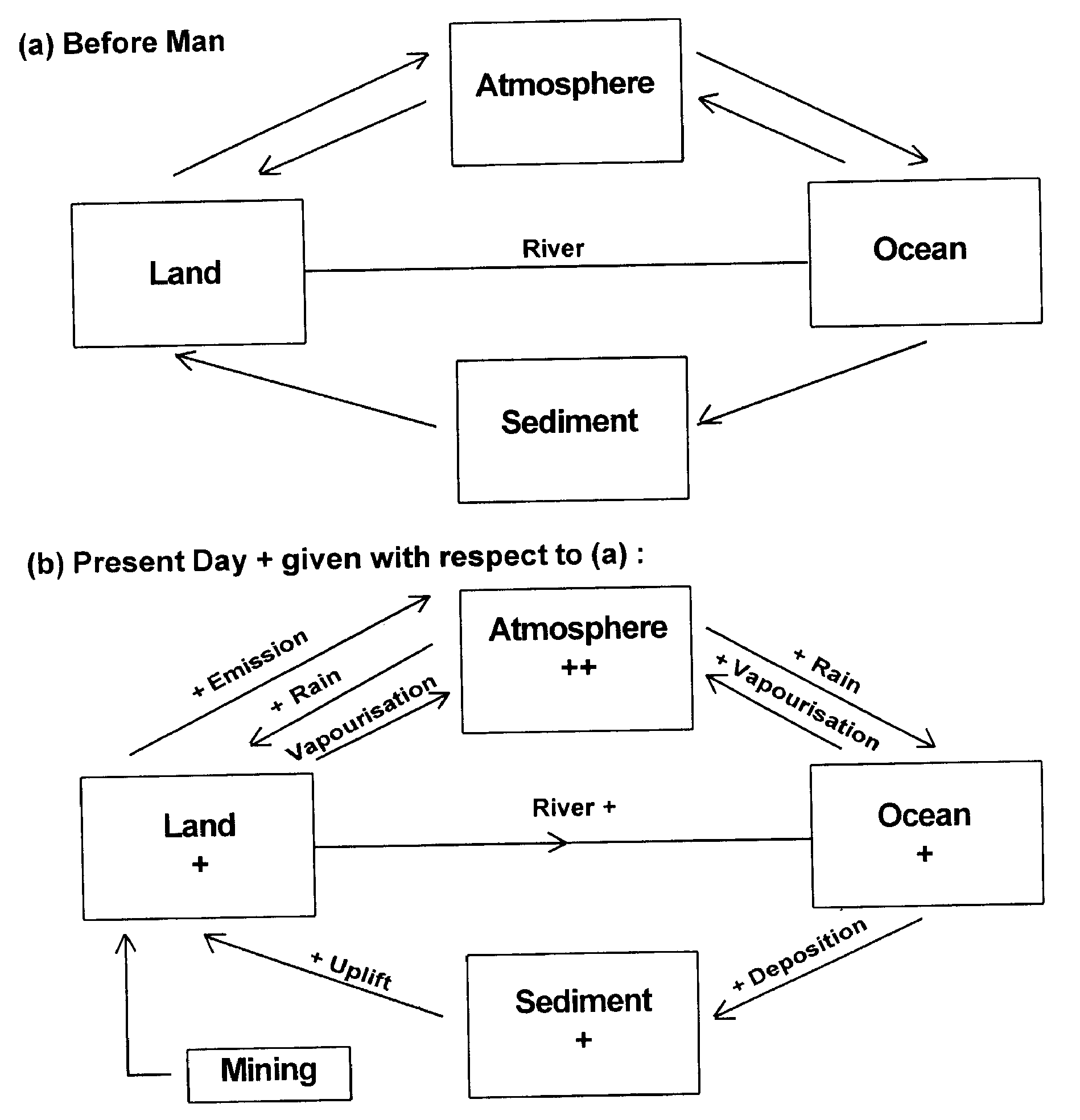
Hg cycle is shwn in tow stages: pre-man and present day. this concept is adapted from Garrels et al., 1976. The main differences is indicated in teh Hg - mining in the present day cause additional flux - vaporisation - from land to atmosphere. ++ or + singns indicate higher gains in the respectinve sectors i the present day cycle relative to pre-man cycle. As a consequence there is also poportional increase in the flxes in the present day cycle between the concerned sectors. Garels et al. (1976) estimate that Hg will be the first metal that is likely to be lost out entiely ot of the solid spheres (litho - and pedospheres) to the fluid (hydro- and atmo-) spheres in foreseabe future.
RESEARCH APPROACHES IN BIOGEOCHEMISTRY
In the previous section we have discussed the basic concept of Biogeochemistry and evolution of this special discipline over the last few decades. As was mentioned in the earlier section, Scientists in the various countries have adopted diverse approaches in solving problems associated with Biogeochemistry.
In the International Scientific World, the present knowledge on biogeochemical cycle shows several approaches and gaps to be filled and also the recommendations in the years to come. These are adapted from several reports of the IGBP (International Geosphere Biosphere Programme)
This can be summarized as follows:
I.1 Fluxes: The Fluxes Can Be Grouped as -
Relatively better known - DOC, DON, DP, DS; (D- Dissolved, O- Organic, S- Solids)
not well known - POC and hence TOC; (P- Particulate, T- Total)
characterization and fluxes of individual components of DOC (such as lignin, amino acid) are not known;
essential trace metals such as Fe, Mn, Cu etc. - their total concentration and speciation both in dissolved and particulate form need to be known in detail;
effect of natural (catastrophic events such as floods), or man-made (dams, diversion of channels) impact on fluxes;
diverse sources of C, N and other elements, i.e., natural vs. anthropogenic, riverine vs. atmospheric and their relative contribution to global fluxes;
present flux rates need to accommodate newly available data from the USSR and other regions for which data are available.
Processes:
certain microbiological processes such as heterotrophic systems need to be studied better to understand nutrient cycling in micro-ecosystems;
photo-chemical reactions involving trace metals and organics can adversely affect C- availability. We do not know to what extent such processes affect primary production;
interaction at several interfaces such as air-water, water-sediment, sediment-pore water need to be studied to understand C-cycling processes.
Possible equilibrium/exchange between DOC and POC is not well known and kinetics of all the processes in micro-systems need to be understood for elemental cycling;
particle interactions such as solid-solid (flocculation), organics-inorganics may regulate C-availability and hence biological activity in estuaries;
effect of human activities on the fluxes and processes need to be better understood.
In order to close the gap in both with respect to fluxes and processes mentioned above, we propose the following approaches were proposed:
Analytical: Flux calculations and processes evaluation depend largely on methods used to analyze C and other elements; more recent methods of C analysis if extended to all river and estuarine systems, may significantly enhance present state of knowledge on C transport in estuarine and thus marine environments. Analysis of colloids and complex species of trace elements require investigation in the light of C-cycle. Stable isotope techniques may also help in flux determinations and processes studies.
Experimental: In the C-cycle, experiments on essential trace elements and nutrient studies should complement field data. Laboratory scale studies, simulation models of estuaries(large scale - Rhode Island model or small scale - China model) and tracer techniques can promote understanding of estuarine systems.
Small Rivers and Estuaries: They are ideal for detailed process investigations outlined earlier. Both analytical and experimental models can then become viable to interpret elemental transfer within a small system.
Large Rivers: Fluxes are best estimated in large systems globally and efforts fill-up the existing gaps should be made in this regard. Available data on large rivers such as Yenisei (USSR), Ganges (India, Bangladesh) may be integrated influx estimations.
Type-Systems: River and estuarine systems need to be studied from diverse type-areas reflecting geology, climate, physiographic regions, pristine and at various levels of human impact, and flood and draught prone areas. Our present knowledge on most of these controlling factors is inadequate.
Models: All flux calculations and controlling factors need to be evaluated with respect to an appropriate modeling.
2. Natural Lakes and Reservoirs
It has become generally accepted that lakes and reservoirs are not a significant term in global the global budgets of biogeochemical elements. The rationale for studying natural lakes and reservoirs consists of at least the following points:
They are essential to humans from the view of water supply, agriculture, recreation, and power generation.
The results of human activities is having an increasingly adverse effect on lakes. Sulfur emission is one important anthropogenic effect.
Acid rain is a serious problem in some classes of lakes.
Furthermore, subtle effects of sulfur additions affect other classes of lakes. Lakes are an excellent environment to record changes in global biogeochemical cycles and climate.
Lakes are excellent sites to study processes that are important in other aquatic environments (marine).
Lakes ar significant habitats for biological diversity (preserve endemic species - Lake Baikal, Australian Salt Lakes, East African Rift Lakes).
Biogeochemical cycles of C, N, P, S, and trace elements are strongly interconnected and should not be studied in isolation.
Interactions between Sulfur and Carbon:
The effects of acid sulfate precipitation on lakes of low buffering capacity are now well known, and it is clearly established that quite a number of lakes have been adversely affected. However, sulfur additions to lakes can have consequences well beyond those due to acidity, and even large numbers of lakes can thus be affected in relatively subtle ways. In Figure - 5, we outline some of these other effects of sulfur on lakes, emphasizing some direct and indirect effects on carbon cycling.
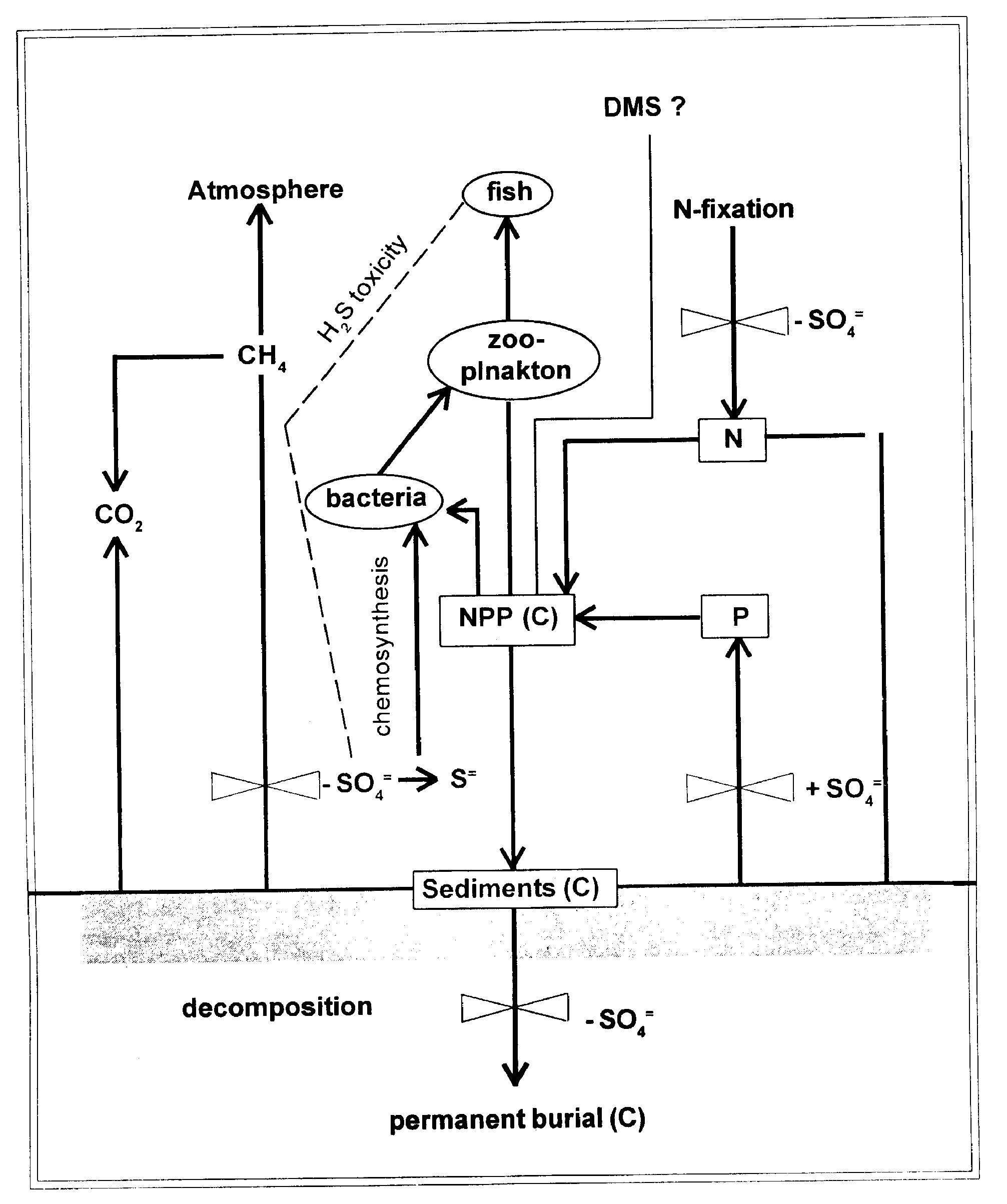
Effect of Increased Sulfate on H2S Toxicity:
Sulfate reduction in lakes is generally limited by sulfate concentrations. Thus, if sulfate concentrations are increased, sulfate reduction rates will increase, and the potential for hydrogen sulfide to occur in the water column also increases. Hydrogen sulfide is toxic to a variety of organisms, and in lakes where physical conditions allow this sulfide to accumulate in the water column, consequences can be severe.
Effect of Increased Sulfate on Phosphorous Cycling:
Primary production in most lakes is limited by phosphorous availability, and so any change in phosphorous cycling will change primary production. Increased sulfate concentration may increase the release of phosphorous from lake sediments perhaps by reducing the amount of sorptive iron oxides and hydroxides in the surface sediments. Thus, increased sulfate inputs may cause further eutrophication.
Effect of Increased Sulfate on Nitrogen Fixation:
Nitrogen fixation can also be affected by increased sulfate concentrations, at least if concentrations are increased sufficiently. This because sulfate inhibits the uptake of molybdenum by microorganisms, and molybdenum is required for nitrogen fixation, an effect of higher sulfate concentrations may be to alter nutrient limitation from phosphorous to nitrogen.
Effect of Increased Sulfate on Carbon Burial:
The extent of anaerobic organic carbon degradation in sediments appears to be greater when sulfate serves as an electron acceptor (sulfate reduction) than when decomposition proceeds throughmethanogenesis. This is perhaps one reason why the organic carbon content of the sediments of Lake Baikal (where sulfate concentrations are relatively low) is high in relation to comparable marine ecosystems (where sulfate is much higher). Consequently, increasing sulfate concentrations in lakes may reduce the burial of organic carbon in sediments.
Effect of Increased Sulfate on Chemosynthesis and Anoxic Photosynthesis:
Chemosynthetic production and photosynthesis by anaerobic bacteria using sulfide as an electron donor are significant sources of organic matter production in some lakes. Since:
The productions by anoxic photosynthesis and by chemosynthetic bacteria to increase as sulfate inputs into lakes increase are expected.
Effect of Increased Sulfate on Methane Flux to the Atmosphere:
The flux of methane from lake sediments is the difference between the rate of methane formation and methane oxidation. Increasing the sulfate concentration will both lower the rate of methane formation and increases the rate of anoxic methane oxidation. Thus, increasing sulfate concentration should greatly reduce the flux of methane from sediments. We recommend studies of the interaction of sulfur and carbon in a variety of lacustrine environments. We need to focus future research on interconnections that are not well understood (Fig. 2). While we recognize that there may be effects of the carbon cycle (mass) on the sulfur cycle, the strong effects of sulfur on carbon suggest that we concentrate our limited resources on these interactions.
Figure - 6 : The effects of lake parameters on the interaction between sulfur and carbon.
-------------------------------------------------------------------------------------------
PROCESS sediment sediment nitrogen sulfide methane chemo- bacterial
decomposition p-release fixation toxicity flux (pro- synthesis photo-
(carbon duction, synthesis
FACTOR burial) oxidation
-------------------------------------------------------------------------------------------
1. Physical
depth + + + + + +? +++
stratification + + + ++ + + +
turbulance + +? + + + + +
residence time + + + + + + +
cold vs. warm ? - + + ? + +
natural vs.
man-made ? + - - - - -
-------------------------------------------------------------------------------------------
2. Chemical
salinity (hydro-
chemistry) +? +? - + + or - + +
buffering cap. -? -? - ++ - + +
mineralogical
composition +++ +++ - + - + +
-------------------------------------------------------------------------------------------
3. Biological
productivity
gradient + - + + + + +
community
structure - - - + - + +
-------------------------------------------------------------------------------------------
+ = effects
- = no effect
The physical, chemical, and biological characteristics of a lake can greatly influence the relative importance of particular interactions between the sulfur and carbon cycles. We have tried to emphasize this by preparing a matrix of the effect lake-type factors on the interaction between sulfur and carbon (Fig. 2). In this matrix, a + sign indicates that whether or not increasing sulfate affects the particular C-S interaction as a function of the lake type. A - sign indicates that the lake type does not influence the effect of increasing sulfate concentration on the C-S interaction. Three + s indicate a strong interaction, whereas question marks indicate uncertainty as to a possible interaction. The matrix represents consensus of the working group members but is only a preliminary attempt. In fact, there is still a great deal of uncertainty concerning many of the element interactions in lakes and the influence of lake characteristics on these interactions.
As a final note, strong interaction of sulfate with the carbon cycle is related to the mineralogy composition of a sediment.
3. Carbon and Sulfur Cycles in Seas Marginal to Continents
The C and S cycles in regions of the oceans contingent on continental margins (marginal seas) are exemplified by the Black, Baltic and Mediterranean seas, but excluded from our definition were seas such as the Caspian.
The factors influencing C and S budgets in marginal seas. These factors encompass sources, fluxes and fate of these elements. With regard to sources, marginal seas receive C and S inputs from allochthonous and autochthonous means. Major allochthonous inputs include:
With regard to autochthonous, sources include:
Primary production in the marginal seas can be evaluated as corresponding to mesotrophic- eutrophic levels. Under natural conditions it varies within 0.3-1 gm2/a, being composed of phytoplankton production - phytobenthos production in relations 10:1 - 100:1. Main gaps in knowledge are caused by underestimations caused by the use of (14)C-method, missing of seasonal maxima, especially end winter early spring temperate seas, and scarce coverage of areas by stations. Total input of marginal seas into the global production is large and can be evaluated as 25-30%.
Modern anthropogenic input of nutrients creates the tendency of substantially enhancing primary production in marginal seas, especially in coastal areas for several times.
Furthermore, benthonic and microbial activities are correspondingly higher than in open ocean sediments. Sediment mineralization of organic carbon drives the S cycle via sulfate- reduction as well as by sulfate-linked methane oxidation.
Stable carbon isotopes (C13) in organic matter from many coastal/shelf sediments indicate that organic matter of terrestrial origin is deposited nearshore. Further, isotopic data and organic carbon contents of suspended matter and bottom deposits in estuaries and coastal seas indicate a large loss (in the order of 50-75%) of organic matter of terrestrial (fluvial) origin. It is not clear yet as to what extent the (C13) of organic matter is influenced during consumption or alteration of organic matter with an originally isotopic light composition is marked. It is therefore recommended to study the transformation of organic matter using isotope techniques.
The group recognized several ways in which humans impact upon the C-S cycles in marginal seas:
direct discharge of organic matter.,
eutrophication developments by input of nutrients,
destruction of natural ecosystems by activities such as chemical pollution, stimulation of H2S production, mechanical construction, dredging and trawling,
deforestation and irrigation practices disrupting normal terrestrial drainage.
The recognized research needs are :
To acquire better spatial coverage of processes relating to the cycle of S-C in marginal seas. Although some areas are particularly well-studied (e.g., Black Sea, Mediterranean, etc.) a large group of marginal seas remain so far unstudied (e.g., Okhotsk Sea, high-latitude ice- stressed regions).
To intercalibrate the diversity of methods employed to estimate primary productivity, sediment/POC flux and microbial mineralization reactions. This is especially important for biological radio isotope tracer investigations.
To make comparative studies of the C/S cycles between pristine and polluted ecosystems in order to get an insight into the impact of human activities.
To pay particular attention to tropical marginal seas because of the fragile nature of coral reefs under threatened existence from deliberate human destruction.
To supplement field studies with laboratory investigations in order to substantiate the findings of the former.
To make comparative studies of marginal seas having different climatic regimes. This will achieve a better understanding of the role marginal seas play in the global environment.
To take an interdisciplinary approach to marginal seas investigations.
4. Air - Water - Life Interfaces
Introduction : The biogeochemical cycling of elements in the atmosphere and in aquatic ecosystems on land especially at the air-water-life interfaces. Emphasis was placed on the disturbance of the natural cycles by anthropogenic activities on the one hand and on the modulating influence cycles of the biota on the other. Sulfur was treated as the reference element.
Consideration of Pathways Between Sources and Receptors
Anthropogenic emissions of sulfur containing gases lead to increasing concentrations of SO2 and sulfate in the atmosphere even in remote areas. The atmospheric sulfur burden is brought back to the earth s surface by dry and wet deposition. Besides their deposition on vegetation and soil, sulfur compounds are also deposited on the surfaces of aquatic systems. The flux of anthropogenic atmospheric S into water bodies can be investigated by using sulfur isotopes provided that the industrial sulfur sources are isotopically different from the isotopic composition in environmentalreceptors. Sulfur isotope data have been mainly used for qualitative interpretation. However, with more effort this technique could also be used quantitatively.
Sulfur Isotopes Have Been Used to Demonstrate:
An increase in sulfate concentration in small water bodies due to SO2 dry fall out and sulfate deposition which originate from industrial sources;
a link between the concentration of dissolved organic sulfur and the proportion of anthropogenic sulfate in small water bodies;
the acquisition of sulfur by emergent plants from both the atmosphere and sediments; hence the decay of plants provides an indirect pathway for atmospheric S to enter the water body; and
that sulfur isotope data for sediments and nearby soil horizons provide a comparison of fluxes to the soil with fluxes to the air-water interface and further downward movement through the water column to sediments.
Processes at the Air-Water Interface
The physical structure of the air-water interface is complex and still awaits clarification. However, with respect to its influence on mass exchange between air and water, existing informations from various laboratory and field experiments allow an empirical description of the exchange flux, it is the product of : (i) the difference between bulk concentration in air and water respectively, (ii) C, and (iii) a coefficient K which has the dimension of a velocity. Generally, K depends on the structure of both the air and water boundary layer. Moreover, in most cases only one of the layers is controlling the flux. The flux gases with large Henry coefficient meaning low solubility (O2, N2, CO2, SO2, DMS, etc.) are controlled by the water layer. In contrast, gases with small Henry coefficients or large solubility (water vapour) are rate-controlled by the air layer.
Both the air- and water-controlled transfer velocities depend on the wind speed above the water surface. Comparison of experimental K-values, determined at the same physical conditions, show that K depends on D" where D is the molecular diffusion coefficient and a coefficient between 0.5 and 1.
A special situation is met for reactive substances if their reaction time is short compared to the time needed for the molecule to cross the boundary. Such reactions may be purely chemical or result from the interaction with special living communities and from organic films often found at the air-water interface.
Water Column Processes
Important regions in which reduced sulfur compounds are produced are the water bodies of marshlands, lagoons, lakes and intertidal areas. These water bodies range in salinity from freshwater to brackish-marine to highly saline and in depth from a few millimeters to several hundred meters.
The upper layer of these bodies is represented by an oxygenic photosynthetic zone which is the primary source of organic matter. Below this follows an oxic zone where organic matter becomes reoxidized by oxygen-respiring organisms. Where organic production exceeds oxidation, an anoxic zone develops. The interface between the oxic and anoxic zones is termed the chemocline. In lakes, this zonation can be stabilized by temperature, and a chemical stratification may result.
Within the sediment of the anoxic zone bacterial sulfate reduction may develop depending on the supply of sulfate and metabolizable organic matter. This process is the principal source of sulfide in the natural environment and in intertidal zones and sulfate-containing stratified lakes may be responsible for mineralizing up to 90% of the organic input.
The rate of sulfate reduction is controlled primarily by the rate of supply of organic matter and may reach 50mg/l per day in shallow saline lakes.
In shallow water bodies and at acidic pH values, hydrogen sulfide may escape to the atmosphere but for other situations it is oxidized in the chemocline by cyanobacteria, green and purple photosynthetic bacteria and colorless chemosynthetic sulfur bacteria. This leads to a secondary zone of organic productivity, and localized matter or, in stratified lakes to a substrate, that acts as a biological filter preventing the loss of sulfide to the overlying zones. In the chemocline zone sulfide becomes oxidized to elemental sulfur which is subsequently re-reduced to sulfide in the anoxic zone by sulfate reducers.
Induced Emissions of Gases from Aquatic Systems
Recent field measurements have shown that reduced sulfur gases are released from the oxic zones of water bodies and wetlands. Emission of DMS (dominant species), DMDS, COS, H2S, and traces of CH3SH were detected. The emission rate depends on temperature, sulfur concentrations, nature of the biological community and trophic status of the water.
In oceans, the DMS is commonly produced by a few algae species. The mechanisms of DMS formation in freshwater ecosystems are still unknown. The decomposition or organic matter has been suggested as a possible pathway. Therefore higher quantities of H2S might also be released from these regions. Based on available data oceans appear to be the major source of DMS to the atmosphere. However in freshwater the decomposition of organic matter may contribute also to the release of H2S and other sulfur gases in large quantities. This contribution may be further accelerated by continuing the input of anthropogenic sulfur compounds into the aquatic system.
The suggested research needs for:
Further verification of source-receptor relationships with the help of stable isotope techniques and improved dispersion models;
improvement of the techniques for direct measurements of fluxes at the air-water interface;
investigation of the influence of living communities and surface films on gas exchange at the air-water interface;
better understanding of the mechanisms of the interaction of carbon and sulfur in the cycling of organic matter in freshwater systems;
a study of the role of vertically migrating aquatic organisms with respect to the gas- exchange at the air-water interface; and
an examination of the possibility that biogenic emission of reduced sulfur gases might be enhanced by anthropogenic input of sulfur in aquatic ecosystems.
5. Interactions of Carbon and Sulfur at the Sediment-water-Life Interface
The biological and biogeochemical processes in the water column and sediments that influence the flux of particulate and dissolved material across the sediment-water interface were discussed in detail. Processes in both marine and freshwater systems were considered. We attempted to integrate what is known on the individual carbon and sulfur cycles to provide a framework for the interaction of the carbon and sulfur cycles within the sediment- water environment.
The primary interaction of carbon and sulfur is during bacterial sulfate reduction. In this microbial reaction, organic matter is decomposed using sulfate as a terminal electron acceptor and hydrogen sulfide produced may react with organic matter to form an organic sulfide phase. This organic sulfur phase has been shown to be an important constituent of lacustrine sediments. In some environments, such as hydrothermal vents, primary productivity based on energy derived from sulfide chemosynthesis may be more important than sunlight-driven photosynthesis.
Recent word has suggested that reduction in anoxic environments may use methane as a substrate. In high sulfur systems, like Mono Lake, complete consumption of methane produced by methanogenic and fermenting bacteria may occur.
Indirect interactions of the carbon and sulfur cycles were also considered. One potentially important interaction in freshwater systems is the uncoupling of the well-known iron and phosphorous cycles by changes in sulfate. Increasing sulfate may cause an increase in the production of sulfide and iron sulfide compounds, via bacterial sulfate reduction. In this situation, iron may not be available to bind phosphate, thereby causing increased flux of phosphate to the water column with attendant effects on primary production.
Promising issues that should receive high priority for future research are summarized here for two categories: Methods Development and Processes Studies.
Improved methods are required to measure fluxes across the sediment-water interface. Efforts should be focused on automated sensors coupled with data loggers.
Methods for examining the rates of opposing processes should be developed. For instance, net bacterial sulfate reduction is the sum of the sulfate reduction and sulfide oxidation. Separating net sulfate reduction into the two opposing processes may result in an improved understanding of factors controlling sulfur cycling in sediments. Similarly, methods for measuring methane oxidation and methane production could be improved.
Although stable C and S isotopes have been used as a tool in studies of the biogeochemistry of carbon and sulfur, increased use of these isotopes will provide additional evidence on processes and fluxes across the sediment-water interface as well as an overall constraint on the system. isotope fractionation studies under controlled laboratory conditions should also be performed, so that data from processes and fluxes occurring in ecosystems can be properly interpreted.
Standard methods for measuring the various species of sulfur and carbon in sediments should be established, to ensure the comparability of values obtained by investigators. Furthermore, standard reference materials for the stable sulfur and carbon phases should be established and disturbed to assure the quality of measurements.
Better temporal and spatial coverage of processes and fluxes should be acquired. Accurate budgets for the sediment and water column require such detailed spatial and temporal estimates of fluxes. Information on the variability of processes in space and time may lead to improved understanding of factors affecting these processes. Seasonal studies are essential for regional and global budgets.
Although much work has recently been performed 1 on sulfur species, in the sediment subsequent to bacterial sulfate reduction, we still do not have a complete understanding of the factors controlling formation of these species. Additional work on the formation, stability and microbial mediation of organic and inorganic sulfur species plus hydrogen sulfide is required.
Interactions of the sulfur and carbon cycles with other element cycles require further attention. Examples are:
Interactions or the S/Fee, S/N, and S/P cycles. uncoupling of the Fee/P cycle may have direct effects on primary productivity.
Biological and biological reduction of elemental sulfur (S )
Sulfur reduction at low (< 30 Amyl L(-1) ) sulfur concentrations, must be considered.
Decomposition and recycling of organic matter of the sediment-water interface. This zone is known to be the site of large changes but has defied careful study due to sampling problems. The recent development of microelectrodes and samples capable of high depth resolution provides tools for new investigations of this zone.
Modified systems, which also provide opportunities that should be pursued. Examples include experimentally manipulated systems as well as systems modified by anthropogenic pollution.
Satellite images show qualitatively the global distribution of ocean, land surface and atmospheric properties. Besides the observations of distribution patterns, the quantitative retrieval of properties, such as chlorophyll and other water substance concentrations or land biomass, are needed to estimate local and global distribution and fluxes of carbon and sulfur. To accomplish this, algorithms are required to transform satellite observed radiances into estimates of these properties.
For the observation of ocean color the Coastal Zone Color Scanner (CZCS) was the only instrument in space, which successfully observed the chlorophyll distribution a global scale. The accuracy of these estimates are within 30% for open ocean water. Up to now global maps of chlorophyll concentrations are available for 1979-1981. Processing of CZCS data for 1982-1985 is continuing.
Coastal waters, often containing several types of water substances, are more difficult to resolve in terms of chlorophyll concentrations. Even under the best conditions separation of the individual water substances, such as chlorophyll suspended matter and yellow substance can only be achieved to within accuracy of a factor of two.
Qualitative agreement between CZCS and shipboard measurements has been shown for large lakes, such as Lade Baikal. Since 1985 no system equal to CZCS is operating in space. However, for turbid waters such as those found in coastal regions, large rivers and lakes, the Cosmos and Landsat satellites can be used to study small scale phenomena. Because of the low radiometric and spectral resolution of the instruments on these satellites the estimates of water concentrations are less accurate.
Present technology permits the resolution of narrow spectral features, such as the sun- stimulated chlorophyll fluorescence. From shipboard and aircraft multispectral measurements with spectral resolution of 5 nm it is found that these measurements enhance the accuracy of the separation and estimation of different types of water substances.
Groundtruth or contact measurements are necessary for the evaluation and verification of new remote sensing techniques, as well as providing for detailed properties of water substances substantial for ecological studies. Present fluorescence methods are widely used for phytoplankton chlorophyll estimation and its physiological state. Furthermore, the chlorophyll fluorescence induction has been used to separate different dominating phytoplankton species. With the help of luminescence measurements the spatial and temporal distributions of phytoplankton and dissolved organic matter are observed.
The suggested research needs for:
To consider the usefulness of results obtained from remote and contact optical methods for thethematic mapping of regional water surfaces.
To give attention to the possibility of using remote and contact measurements for the detection and estimation of anthropogenic influence on drainage areas.
To consider that intensive theoretical and experimental studies of primary and secondary hydro-optical patterns are necessary for adequate interpretation of remote sensing data.
To consider, as most important task, the necessity of employing three levels of measurements, namely, ship, aircraft and satellite, for water bodies having great ecological interest. These measurements are useful in the development of adequate and temporal calibration of satellite and contact measurements.
To consider the initiation of international complex experiments in the framework of SCOPE/UNEP activities for water bodies important for ecological investigations.
To consider as an important improvement in satellite observation the launch of a high resolution multispectral radiometer providing narrow band measurements in the spectral region of 400 to 800 nm for estimates of ocean color and atmospheric properties.
7. Modelling of Integrated Biogeochemical Cycles
Concerning global cycling of either S or C, etc. nor cycling within an individual water body, modelling concepts were addressed in more general terms. The term modelling is used in many ways. In general, it is a description of a real or hypothetical system. Models serve as a tool for solving specific problems. Therefore it is necessary to properly define the problem before choosing a model.
There are two basic approaches.
Models may comprise statistics, budget calculations, dynamic simulation, etc. They may be physical, chemical, biological, economical, or any combination thereof e.g. an ecosystem model.
Models can be used to solve problems at different levels of complexity. There is considerable controversy as to the complexity required to solve a particular problem. In the interest of efficiency, one should start with the simplest approach that is consistent with the objectives. In the course of applying this model, an extension may be necessary.
Ideally, a model should be capable of prediction. However, it can also serve the useful purpose of integrating information from different disciplines and in turn provide direction to the individual disciplines. Therefore, it is important that there are continuous interactions between theconstructor of the model and the researchers providing the inputs.
Most papers presented at this working group focussed on the mathematical aspects of modelling. Therefore, the discussion was directed more towards the appropriateness of specific equations and mathematical approaches rather than towards applications to S and C cycling. One presentation described computer modelling packages that were particularly accessible to researchers not proficient in computing techniques.
Models more directly related to C and S cycling were presented in other working groups.
As models are tools for solving different problems, it is recommended that concerning the cycling of elements, modellers should also given importance to other subject categories.
It will be of interest to look into the recent publications on Biogeochemistry with relevance to India as well to the global scenario. Gupta et al., 1997 (Biogeochemistry, V. 38, pp 108-127) reports on the nature of organic matter in the sediments carried by the Godavari river and discusses the signature of human impact on the organics. This is for the first time such an article appeared in an international journal of repute directly in this subject matter. Several other papers by our group are in press in similar journals and several related articles are frequently being published by us as well as a few other workers in the field. Ramanathan et al., (1997) published a brief article on the Biogeochemistry of mangroves in Tamil Nadu in the Indian Journal of Marine Science. In an earlier paper, Nayak et al., (1989) estimated the carbon cycle in the Indian sub-continent using remote sensing satellite. There are several international and national programmes in witch India have been participating over the last two decades, Mention can be made of SCOPE (Scientific Commits on Problems of Environment, an ICSU Paris based organisation) started a strong international programme on biogeochemical cycles in early eighties and one of the outcome is setting up of an international Carbon research unit at the university of Hamburg, Germany. India was involved in SCOPE projects by organising several international workshops including the Metals workshop in 1987 and Toxic elements workshop in 1989 and late Prof. S. Krishnaswami (former Vice-chancellor, Madurai Kamaraj University) and late Prof. C.R. Krishnamurthy (formerly Director, ITRC, Luckow and later Advisor, Ganga Cleaning Projects) were Vice- Presidents of SCOPE at different times and Prof. V. Subramanian of JNU was the member of the ScientificAdvisory Committee of SCOPE for biogeochemical cycle for metals and for Phosphorous. In fact the Phosphorous programme was initiated by India s efforts in SCOPE. Since late 1980 s, the International Geosphere Biosphere Programme (IGBP) is in place and India is very much involved in such programme (through the active leadership of Prof. A.P. Mitra, FRS and Prof. Daniel) with a thrust on various aspects of Biogeochemistry and again Prof. V. Subramanian of the ENVIS Centre in Biogeochemistry is the All India Co-ordinator of one of the IGBP programmes namely the Biospheric Aspects of the Hydrological Cycle (BAHC-IGBP). Also Prof. P.S. Ramakrishnan is Co-ordinating an International project connected with Soil Fertility in the topics funded by UNESCO/Private agencies.
The ENVIS centre in Biogeochemistry have organised a number of workshops in the last three years and the project staff including the Co-ordinator has given lectures or involved in organising activities at other places such as Children s Science Congress in Delhi, Guahati, Hyderabad etc., special lectures at Coimbatore, Agra, Baroda, Bhubaneswar, Berhampur, Guahati, Imphal etc. A key note talk by Prof. V. Subramanian for the Indian Science Congress in Hyderabad is due in January 1998 and he is scheduled to talk on environmental data base for air and water pollution in a National Seminar at Goa to be organised by Planning Commission in January 1998.
